Water Molecule#
Introduction
Intro paragraph
Notes
To Do
Think about coherent plan
Implement
Colaboration
Star formation is not my expertise so if you want to help, feel free to comment the contribution you could make
Introduction#
Water is … very important:
Solar radiation absorbed by the Earth atmosphere is mostly due to water vapour absorption. []
Key molecule for life …
It is commonly stated that the presence of liquid water is a necessary condition for the appearance of life:
Hydratation properties for biological macromolecules
determine their 3D structures and biological functions in solutions (sol-gel phase transition ?)
…
Water is more than just a solvent
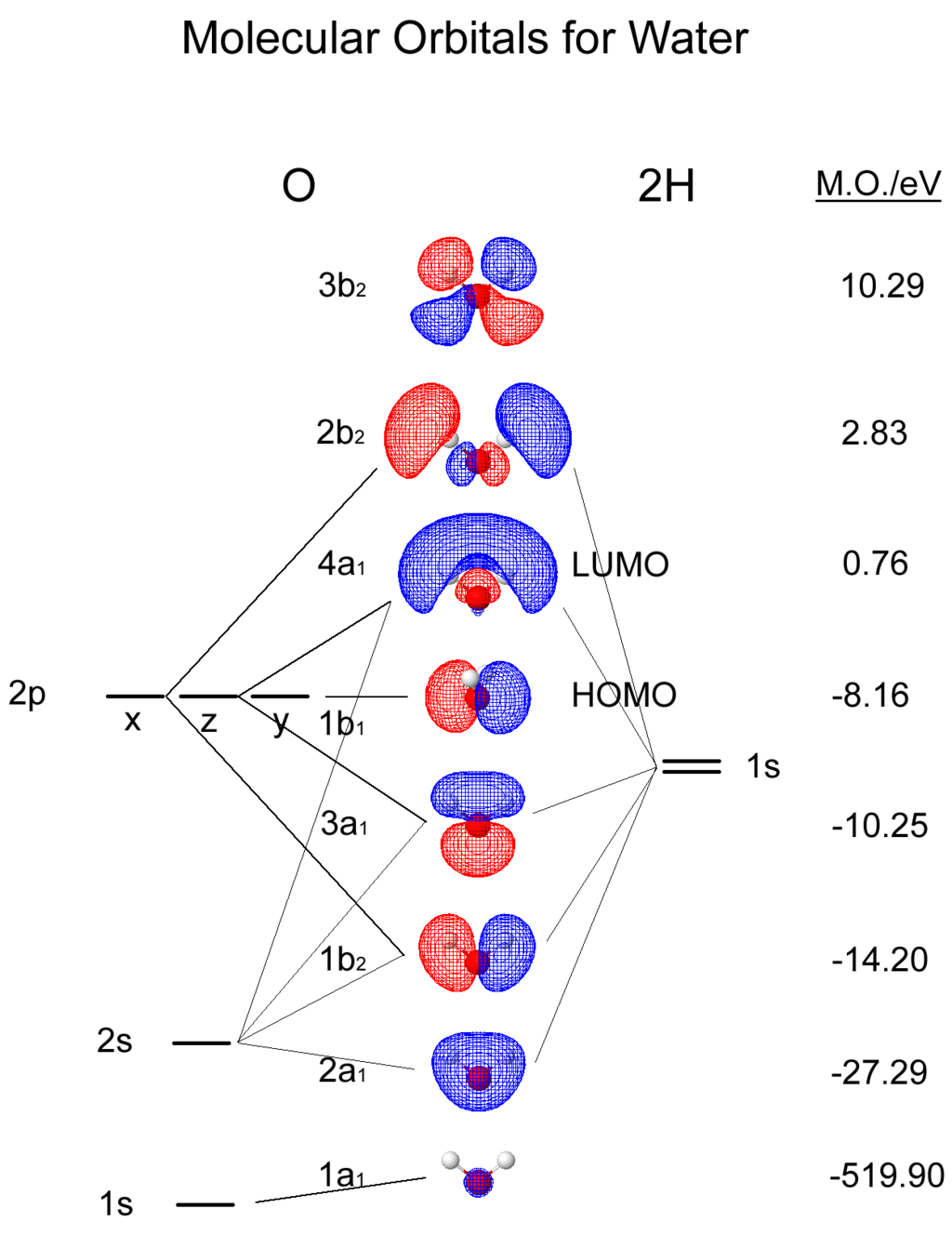
Molecular Structure#
Atomic Orbitals#
Note
Table about the different quantum numbers
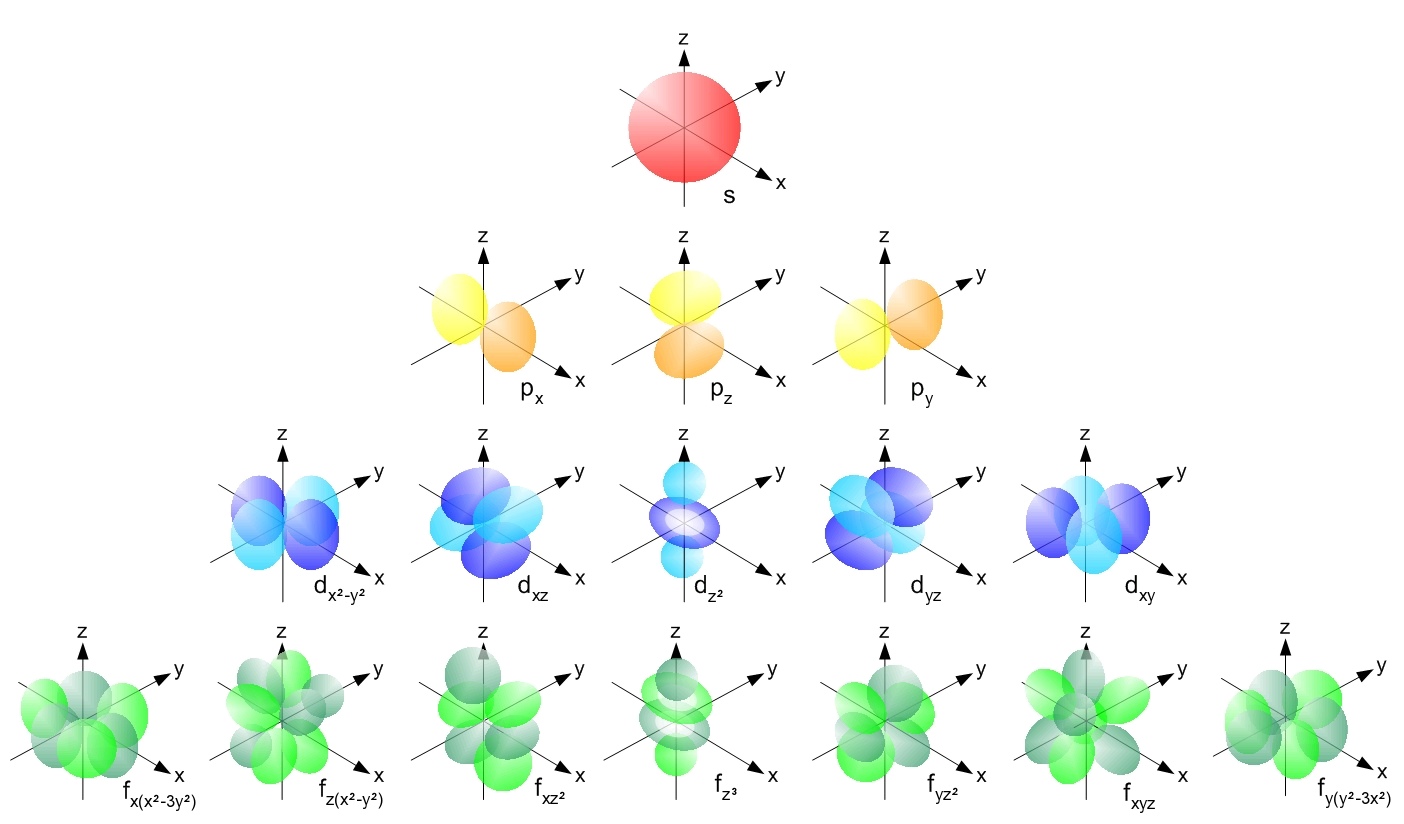
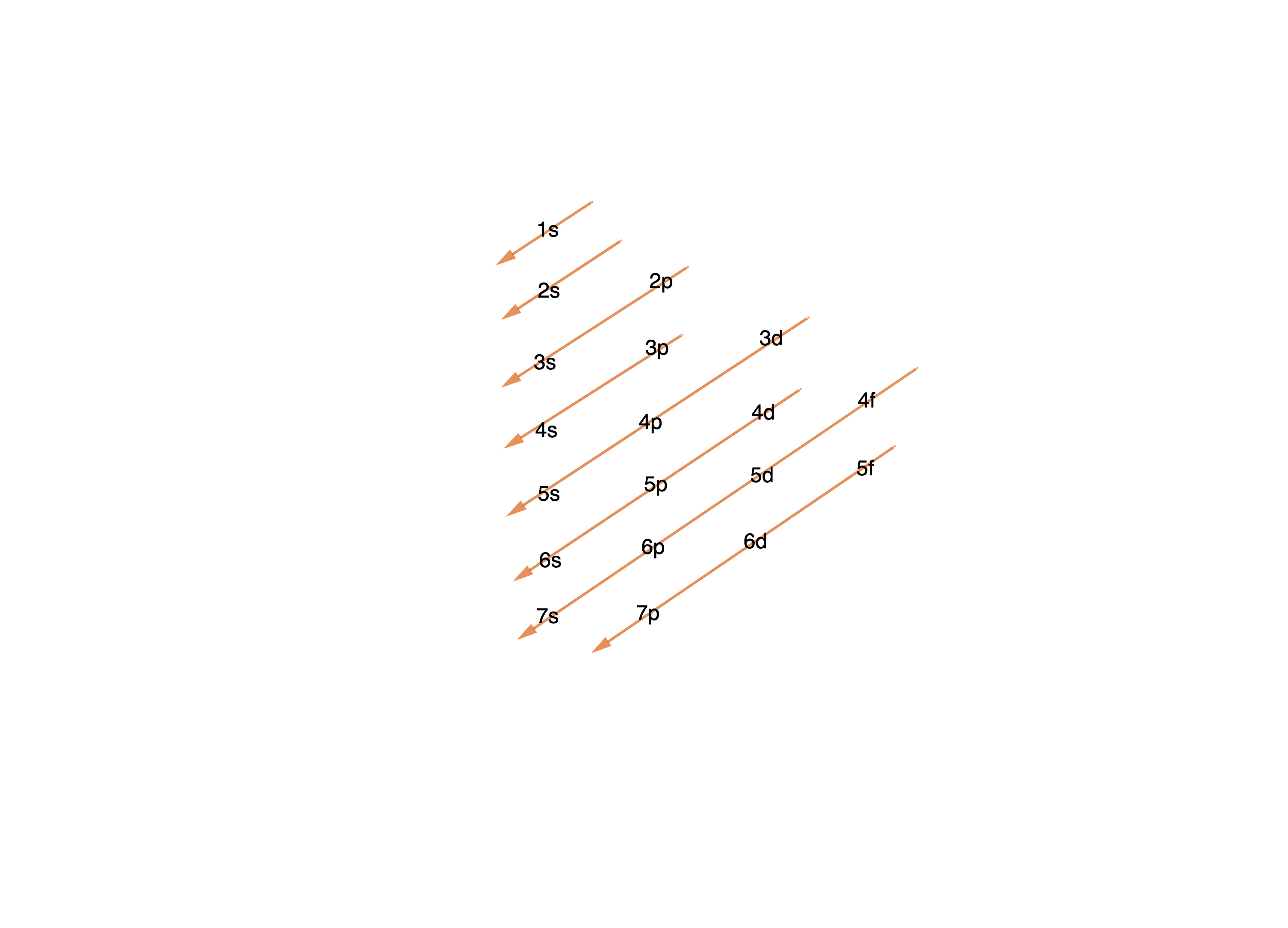
‣ Properties
#‣ ‣ Anomalous macroscopic behaviour
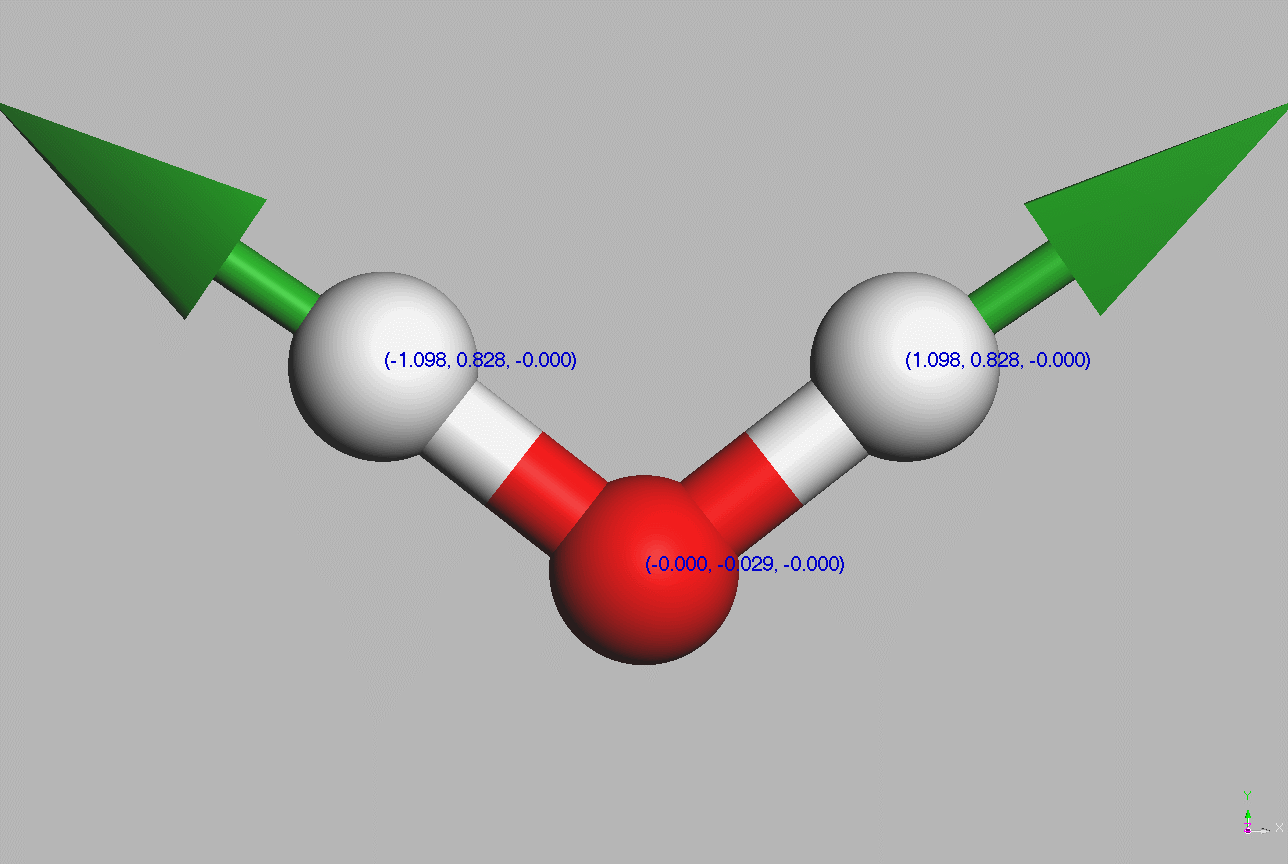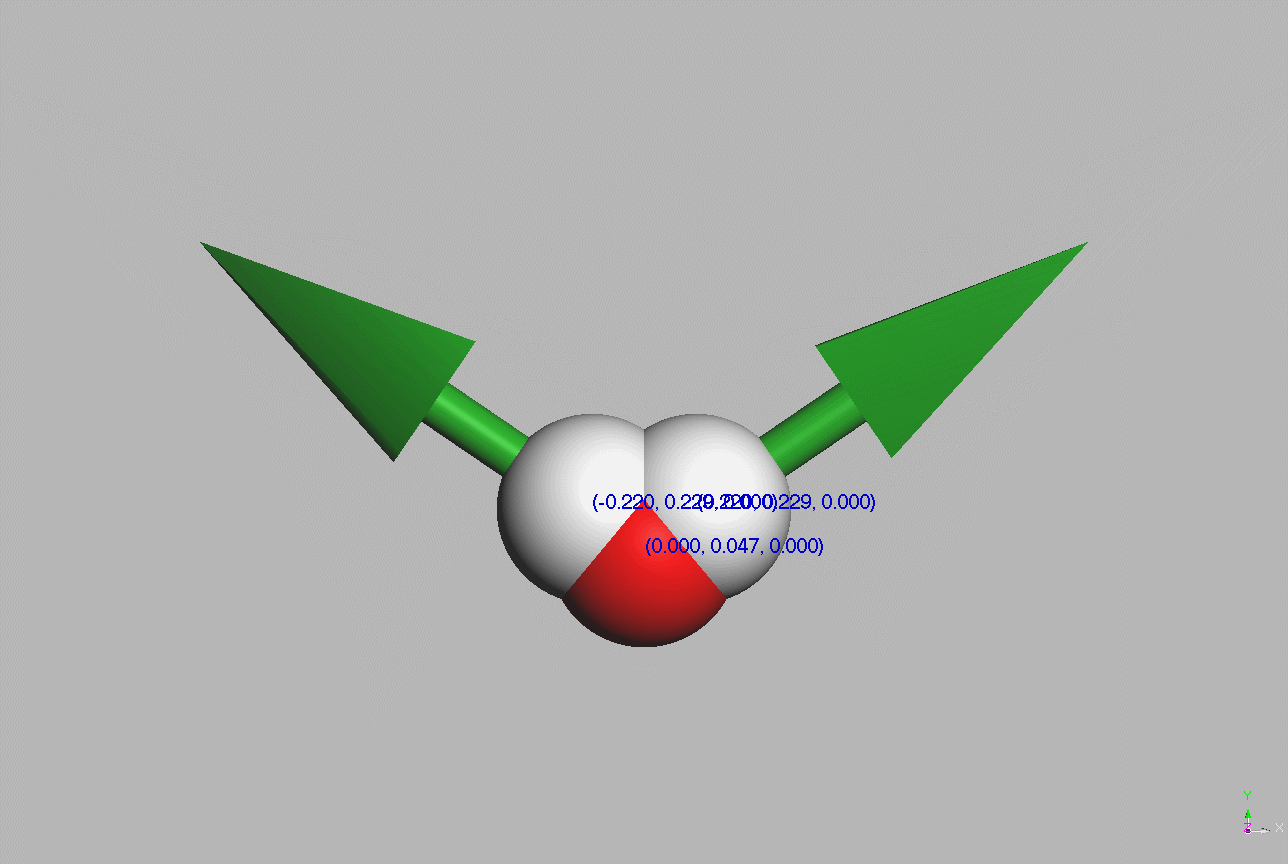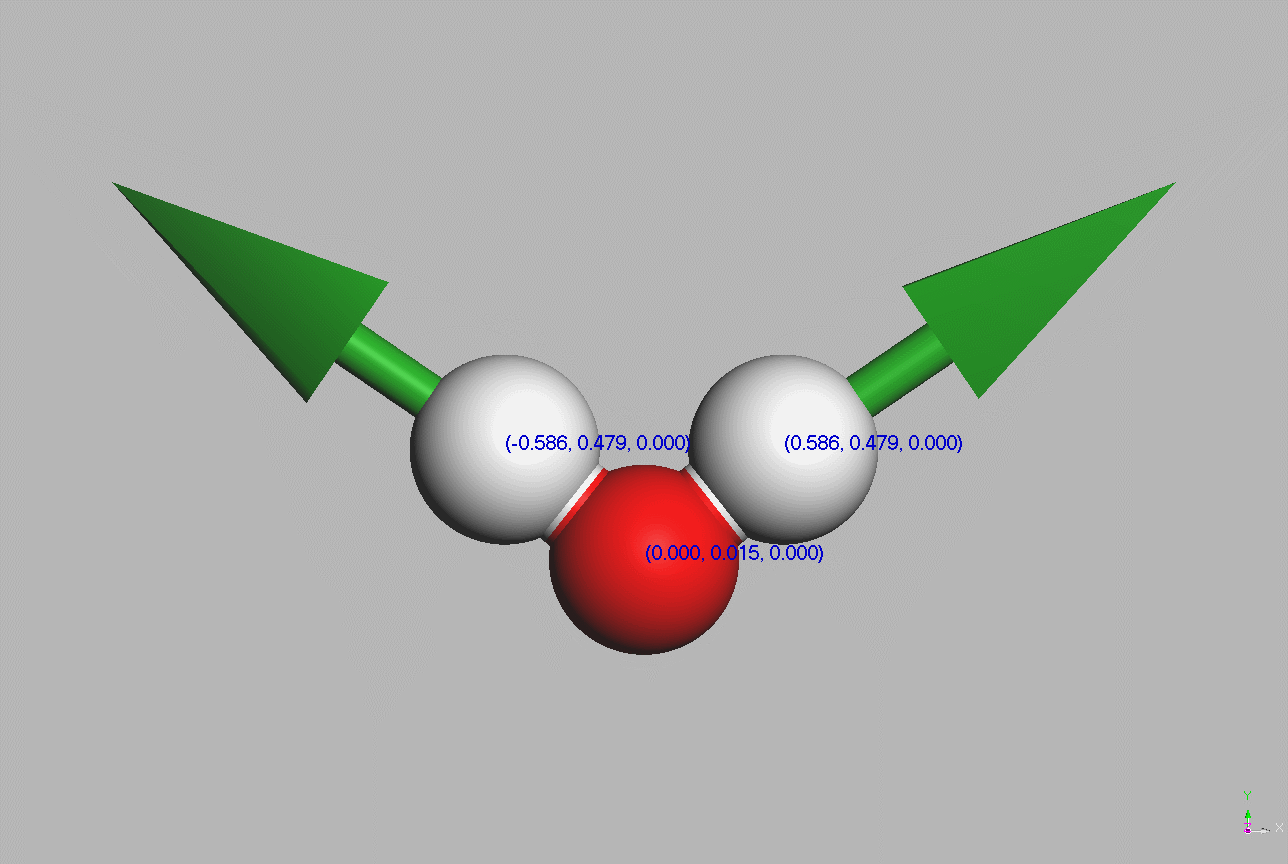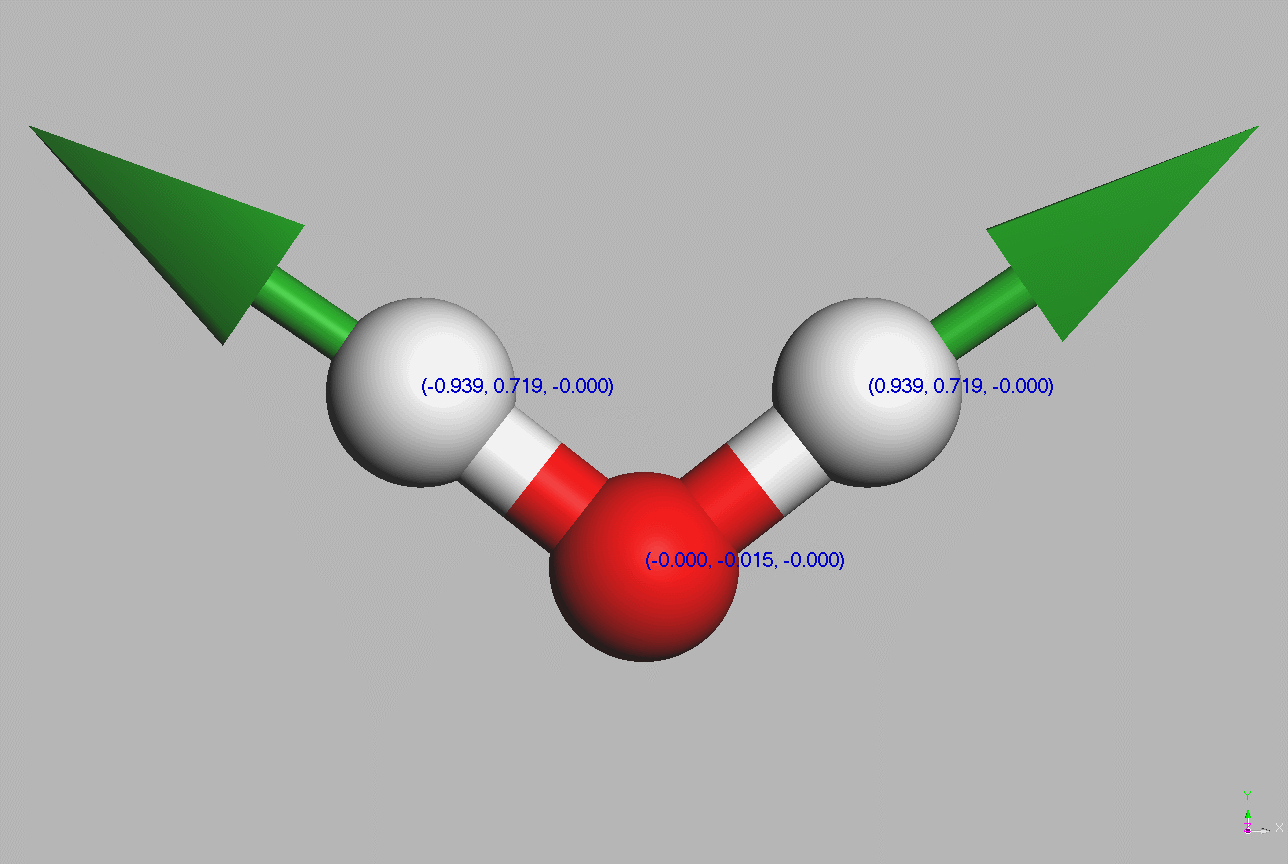
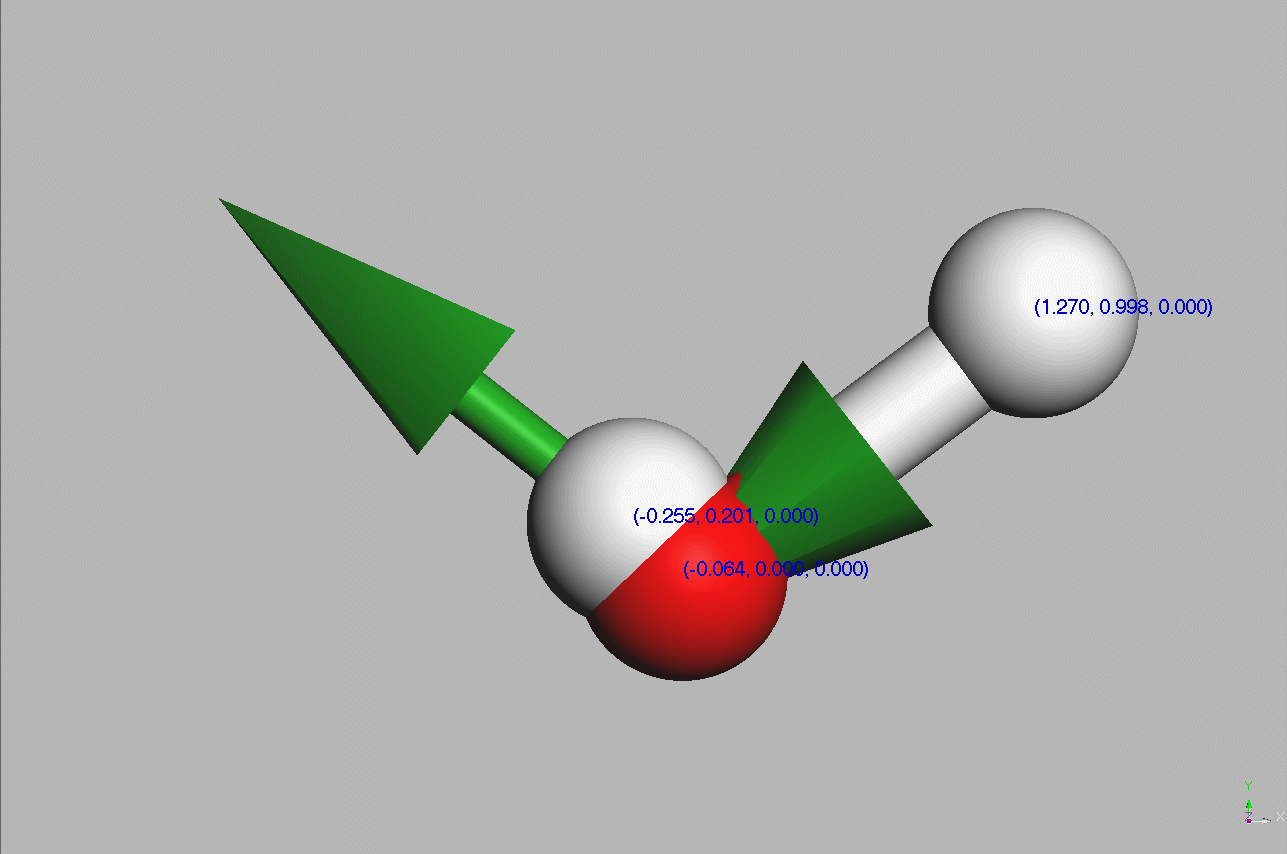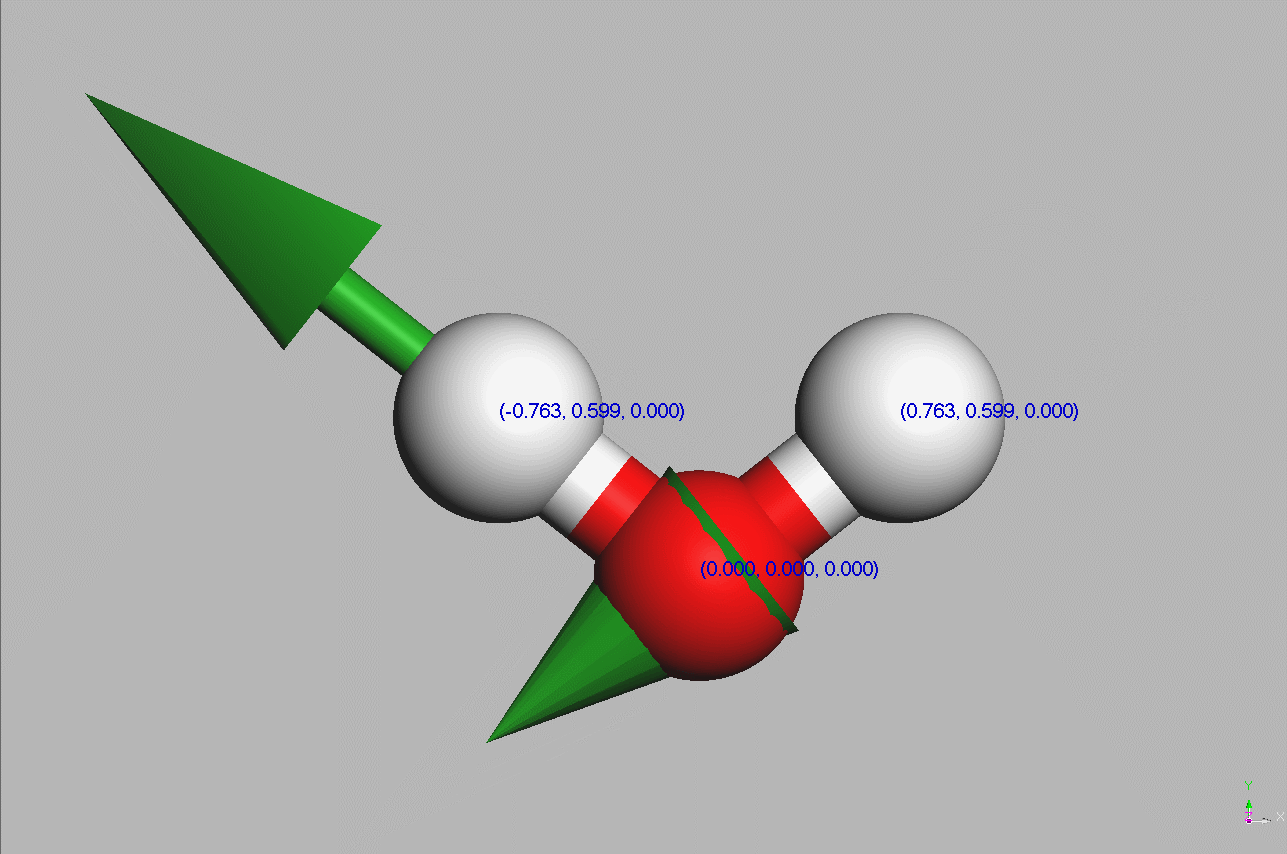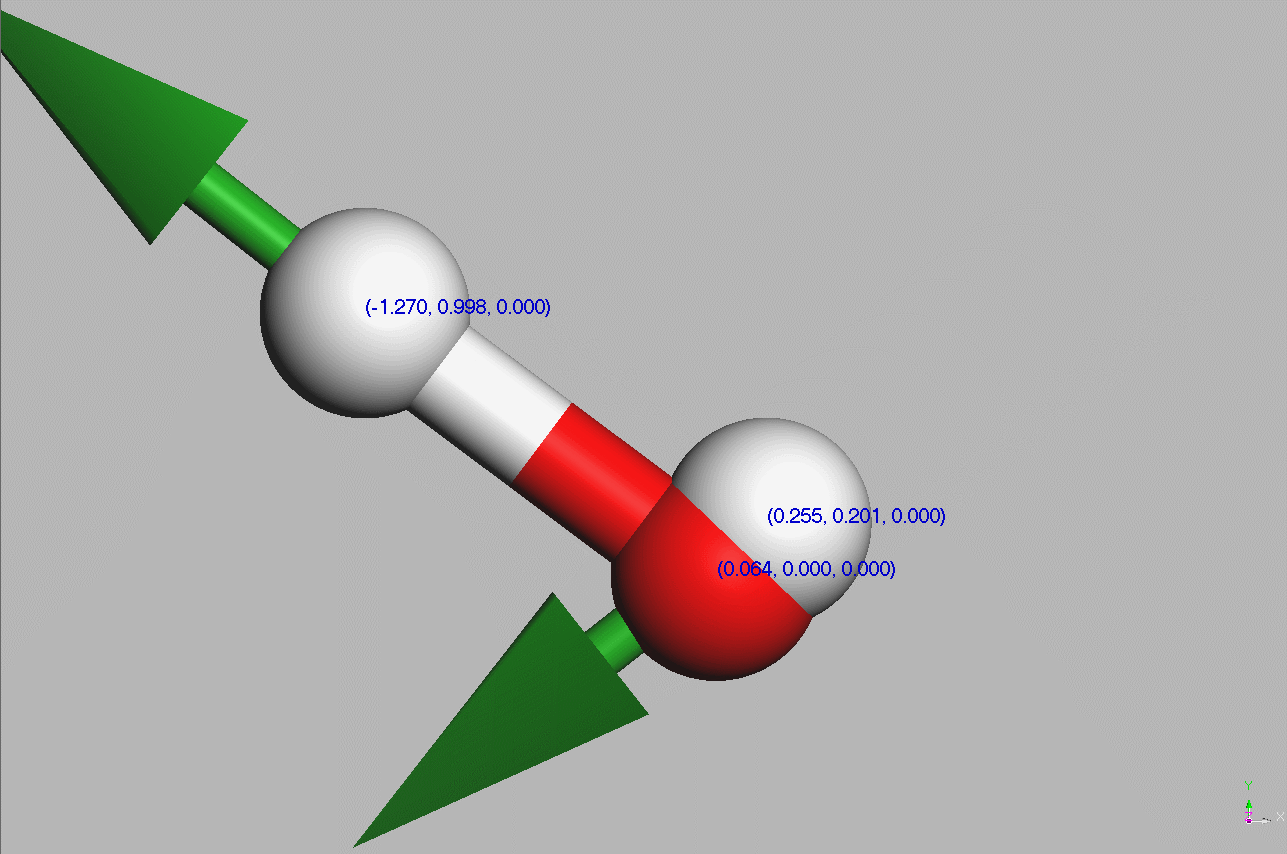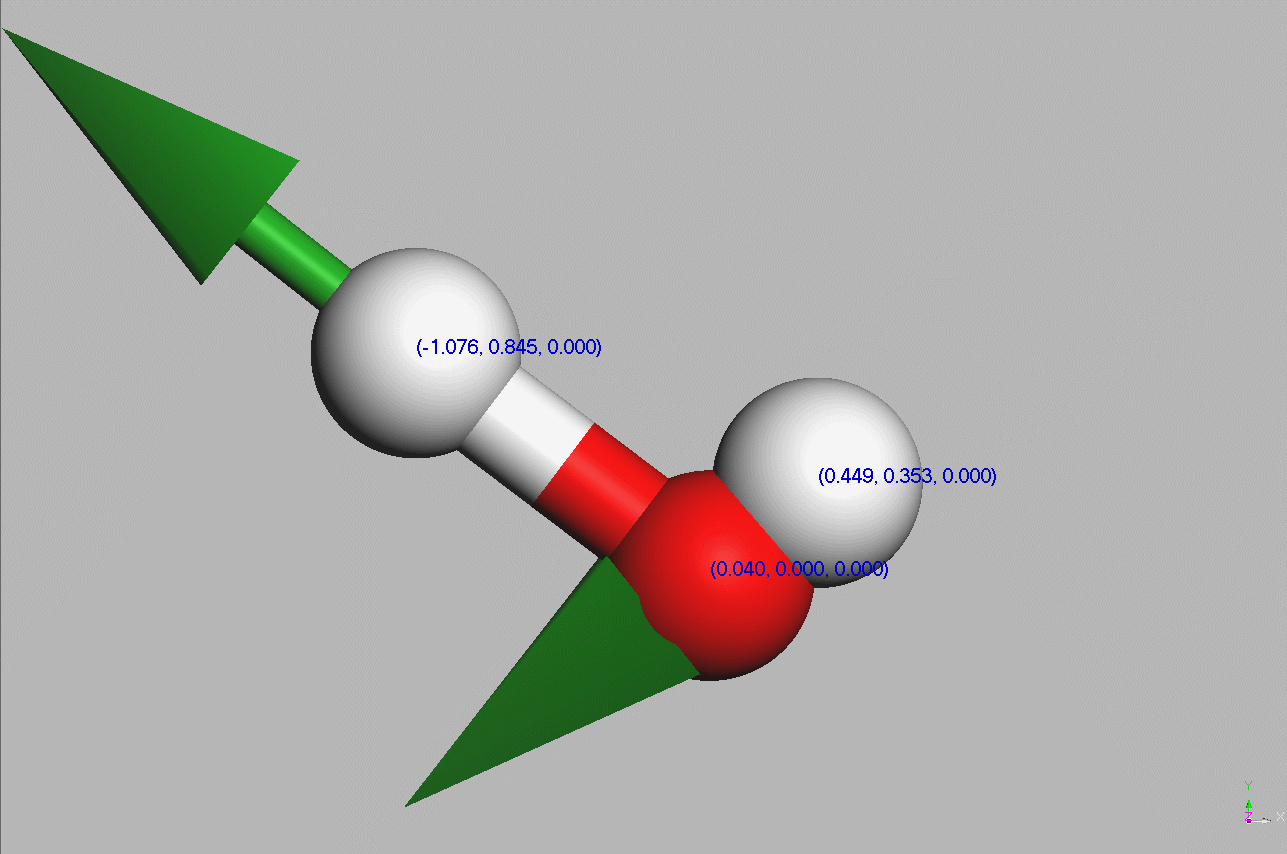
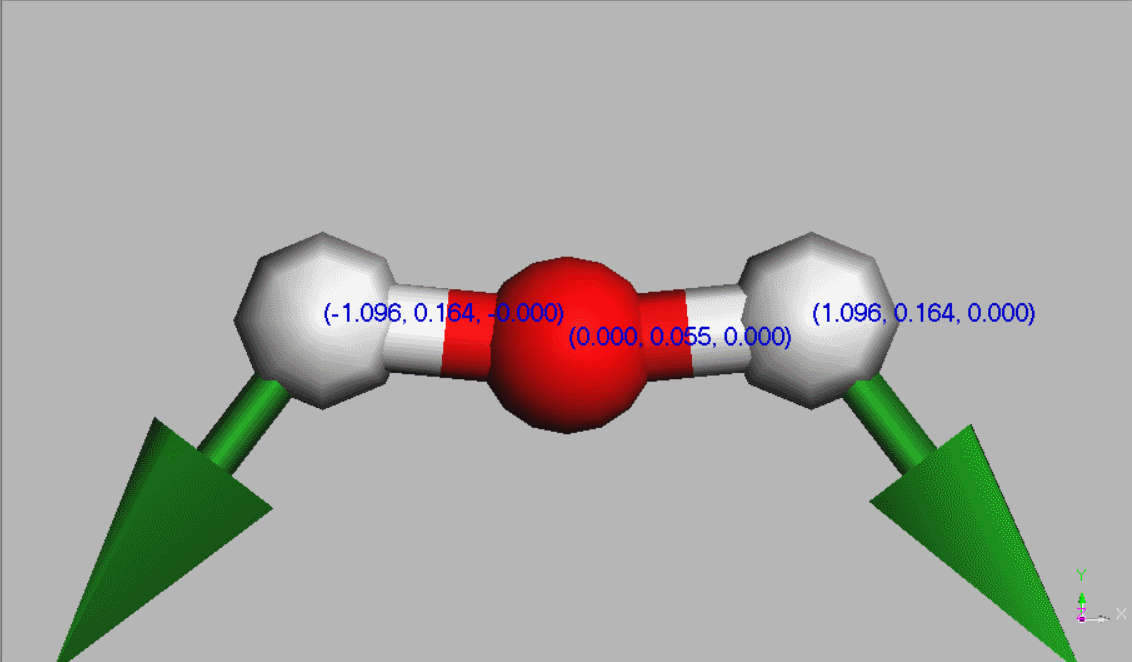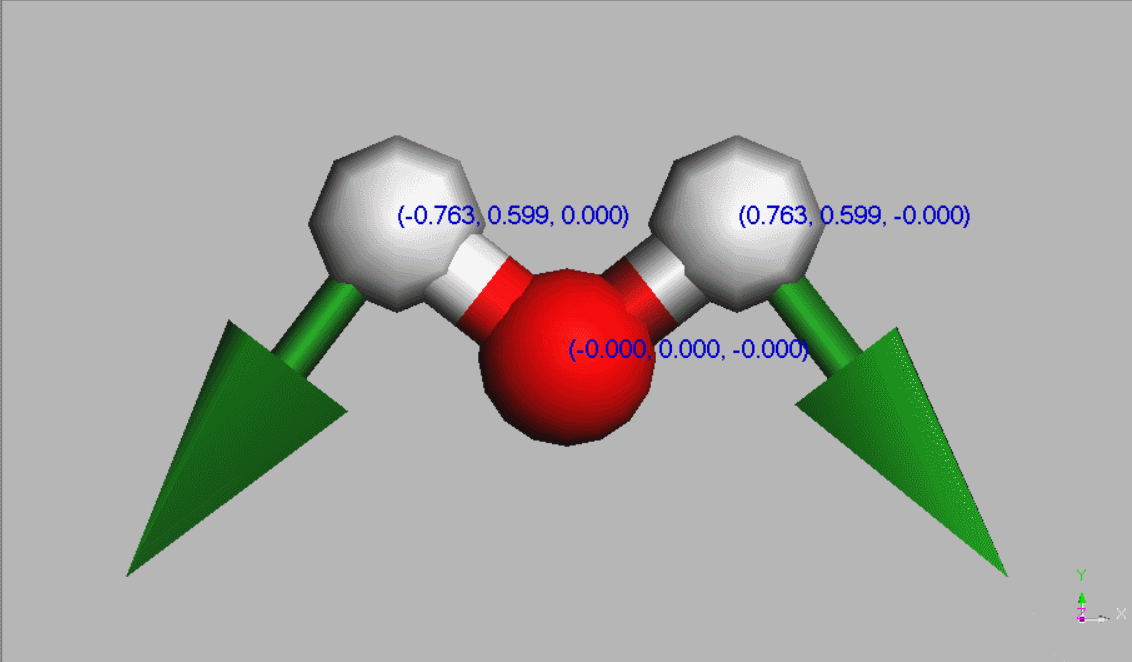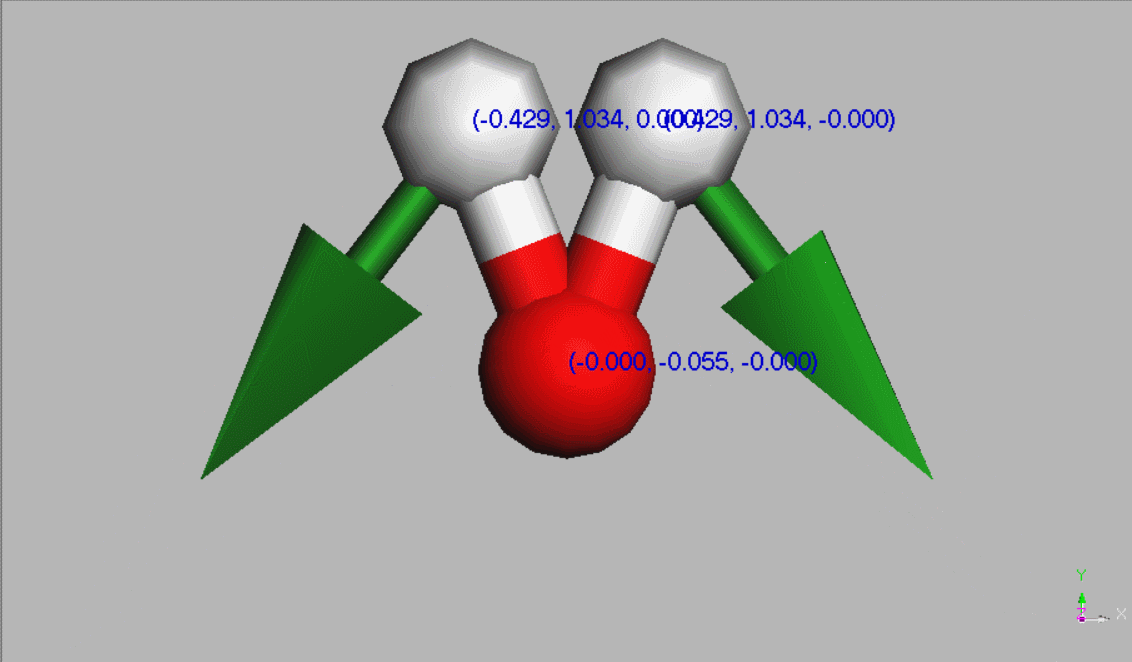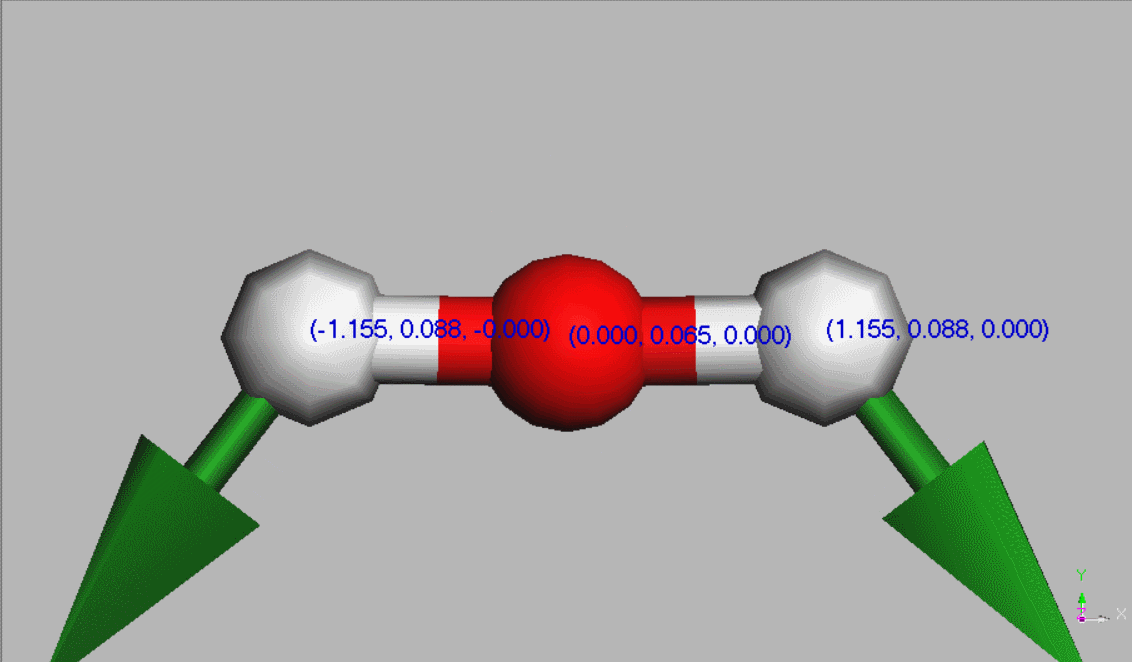
#Water molecules are symetric (point group C2V - two mirror planes of symmetry and a 2-fold rotation axis).
Is it important ?
writing ✏
Geometry,
H atoms could have paralell or antiparallel nuclear spin (orto vs para)
OK, why bother ?
Microscopic properties
Polarity, Dipole moment and Vibrational modes
Polarity makes water an excellent solvent
The dipole moment of a free water molecule is 1.86 D (Debye) [Aida and Akase, 2019] (ref 22) - [Akase and Aida, 2014]
How is it measured, calculated
Monomer band assignment from [Tennyson et al., 2013]
Isotopologues
Isotopologues are a great investigation tool to unveil the various processes that a molecular species has had to overcome over it’s existence.
Hydrogen bonding
Definition
The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.
—IUPAC definition, 2011 (to cite)
Hydrogen bonds are the most important stabilizing interaction in nature, playing a key role in the structure of proteins and DNA. First described by Linus Pauling in a letter to William Bragg in 1929.
copy pasted (rewrite)
The potential energy function of the free AH group is thus modified by the potential B; it becomes broader and the vibrational levels become closer as reflected by the shift of the AH stretching band toward lower frequencies shown by infrared and Raman spectroscopy. At the same time, the proton of the AH group shifts toward B and the equilibrium r (A–H) distance increases while the intermolecular R (A.. B) distance decreases to a value less than the sum of the Van der Waals radii of the A and B atoms. The geometrical changes have been detected by neutron and X-ray diffraction methods (1–4).
Fig. 8 source: [Novak, 1973]#
Different types of Hydrogen bonds ?
linear
bifurcated
Molecular interaction
Geometry
Best way to study intermolecular interaction is in the solid phase, at low temerature (reduce molecular movement ….)
The basic principles that govern the arrangement of atoms in water ice are called the ice rules (or Bernal–Fowler rules) and they state that each oxygen is covalently bonded to two hydrogen atoms and that the oxygen atom in each water molecule forms two hydrogen bonds with other water molecules. As a result, each water molecule is embeded in a teraedron formed by four neighbouring molecules, called a Walrafen pentamer
Fig. 9 Explain: source MC#
This tetrahedral geometry is caused by the ability of water to form 4 hydrogen bonds with other water molecules:
2 donors (D) from the 2 hydrogens
2 acceptors (A) from the Oxygen lone pairs of electrons
Different forms of Hydrogen bonds
Van der Walls interaction / distances
Proton order/disorder ?
Intermolecular coupling
Strong / Weak
Effect on the IR vibrations
Note
Does it strenghten, weaken the bonds?
» Investigation techniques
One of the major issue in obtaining a consensus about water structure is the broad range of technique that are used to investigate it’s properties.
Structure |
Methods |
Time and size scales |
|---|---|---|
Instantaneous |
X-ray absorption |
10-15 s, single molecule |
Almost instantaneous |
Infrared, Raman |
10-14 - 10-13 s, single molecule - local cluster |
Vibrationally averaged |
Models, Pump-probe laser |
10-12 - 10-12 s, single molecule - local cluster |
Diffusionally averaged |
Neutron scattering, NMR shift |
10-9 - 10-6 s, local cluster |
Probability |
X-ray diffraction, thermodynamics |
> s, local cluster |
Infrared spectroscopy
Compatible with astronomical observations (cf next chapter - link)
3µ ice band
Note
orto vs para water - lsbcu
IR vs Raman
Note
What is the difference
Fermi Resonnances** ?
Two-dimensional Raman spectroscopy (?) - [Saito and Ohmine, 2006]