Crystalisation#
Introduction
Notes
To Do
Think about coherent plan
Implement
Colaboration
Star formation is not my expertise so if you want to help, feel free to comment the contribution you could make
Key Review to read !!#
[]
Science of crystalisation#
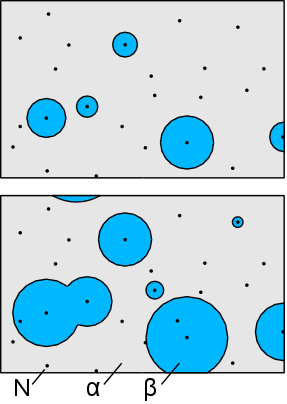
‣ Avrami equation #
‣ Avrami equation #
Also known as the Johnson–Mehl–Avrami–Kolmogorov (JMAK) equation. Describe how solids evolve from one phase to another at a constant temperature. transformation of α into β will proceed by the nucleation of new particles at a rate N per unit volume, which grow at a rate G into spherical particles and only stop growing when they impinge upon each other.
ASW Crystalisation …#
From an Astrochemist point of view
ASW crystalisation is often described as a single event occuring throughout the whole sample
Even though that may be correct within certain laboratory condition, particulary those using high rate of annealing like for example DSC, the picture is probably much more complex than that
Crystalisation Proceses#
Separating nucleation & growth in amorphous ice
- Crystallisation temperature: Tx
- Crystallization rate: RJG
General mechanism: Amorphous ices turning into an ultraviscous deeply supercooled liquid prior to nucleation.
Note
To investigate
Growth#
[]
Models#
Decoupling of simultaneous nucleation and growth processes as well as the quantification of their kinetics []. Growth is isolated from nucleation by dividing a phase transition into two isothermal stages:
- prenucleation, where product crystallites nucleate and grow concurrently
- growth, in which transformation is completed essentially entirely by the expansion of these seed grains
Isothermal transformation kinetics can usually be described by the Kolmogorov–Johnson–Mehl–Avrami equation:
In depth explanation
- Review []
Molecular Dynamics#
{cite:p}`Martelli 2018
Surface#
[]
Substrate#
[]
Crystalisation kinetics of ASW#
The overall kinetics of an isothermal phase transition is usually determined by the time dependence of a specimen’s converted fraction, which is often determined using calorimetry, spectroscopy, or diffraction
Studies that state that ASW crystalises from the surface: []
- insert BD Kay article
ASW vs HGW#
- []: Porosity, SSA & Crystallisation of ASW & HGW: 14 – 150 K, CH4 adsorption -> SSA = 280 ± 30 m2 g-1 ( ASW) and SSA = 40 ± 12 m2 g-1 (HGW), crystallisation rate constant (≈ 7 x 10-4 s-1) independent of deposition T (14, 40, 90 K), hints towards different ASW structure at very low (14 K) T
Operando measurment in order to resolve the ASW crystalisation.
Geometrical vs statistical model#
- []
Glass Transition#
- Modern computational studies of the glass transition Nature Review (not accessible)
Transition between different forms of amorphous ice#
- []: Liquid-liquid transition (TIP4P-Ew model water), 150 – 360 K, low/high/very high density transformations.