Bulk Properties#
Introduction
Notes
To Do
Insert Porosity as a final chapter as it make a good link to surface properties (finish with SSA …)
Think about coherent plan
Implement
Colaboration
Star formation is not my expertise so if you want to help, feel free to comment the contribution you could make
Purity#
ie purely amorphous, no crystaline nuclei
Density#
Hydrogen bonding#
[]
“H-atom dynamics in several forms of ASW, inelastic neutron scattering, mean kinetic energies increase with increasing density (weaker H-bonds) vibrational potential energy surfaces get steeper, change in O-H stretching component with density stronger than suggested by e.g. Raman spectroscopy”
Porosity#
- []: Porosity & SSA of ASW & crystalline ice, N2, CH4 and Ar adsorption, adsorption isotherm volumetry & infrared spectroscopy, non-microporous ice can have large SSA
Evolution with temperature#
Experimental#
IR#
- []: Compaction of ice (H2O, CH3OH, CO2, mixed H2O:CO2 = 2:1) upon heating, astronomically relevant T-range, Laser interference & FTIR, for ASW the full loss of dangling OH bonds is not a proof for full compaction, for other ices thermal segregation benefits from higher degree of porosity.
- []: ASW pore collapse, 20 -120 K, thickness (optical interference) & porosity/phase (FTIR) measurements, porous ASW: thickness decreases by (12 ± 1) % between 20 and 120 K, less porous ASW: smaller thickness decrease, crystalline ice: negligible thickness decrease.
Simulations#
[]
Consequences#
Trapping of molecules#
Warning
Big topic !!
- Find intellegent method of classification … Per molecule studied ?
Note
Link with ethane deposition type
- sequential
- co-deposition
CO2#
- []: SSA of ASW: Spectroscopy on CO2 ice (trapped in ASW pores), deposition at 95 K (simultaneously or sequentially), CO2 infrared bands shift & split in both cases (interaction with water molecules), larger amount of CO2 trapped in ASW in co-deposition, in sequential deposition most CO2 trapped in macropores of ASW, phase transition at 140 K -> CO2 molecules relocate -> similar bulk structure to co-deposited samples
- []: Trapping and release of 13CO2 by porous ASW (13CO2 on top of/below/codeposited with ASW), TPD & FTIR, some 13CO2 becomes trapped when annealing ASW (amount depends on deposition method), two stage release of trapped 13CO2: 1. majority escapes at ASW-to-cubic transition (165 K), 2. rest desorbs together with cubic ice (185 K) -> must be trapped in cavities that do not open during crystallisation.
CH4#
- []: Porosity of ASW via quartz crystal microgravimetry, UV-visible interferometry, and infrared reflectance spectrometry in tandem with methane adsorption: microporosity for all deposition methods, but collimated depositions show additional mesoporosity (up to 140 K (crystallization)), higher binding energy for collimated deposition, methane on dangling OH bonds -> no multilayer condensation inside micropores (methane coating the walls instead of filling the pore volume).
Cracking#
[]
Specific Surface Area#
- SSA (Definition).
Diffusivity#
Molecular diffusion occurs as a result of thermal motion of the molecules.
[]
Models of diffusion#
[]
Desorption#
[Speedy et al., 1996]: Free energy difference (ΔiaG (150 K) = 1100 J/mol) & residual entropy difference (ΔiaS (0) = 0.7 J/(K mol)) for ASW & crystalline ice, from evaporation rates, ΔiaS (0) allows connection of ASW with normal liquid H2O via reversible thermodynamic path (1 atm).
Thermal desorption formula (test)
Polarization#
[]: Surface voltages (Vs) of ASW (vapour deposited below 110 K), Kelvin probe measurements, Vs increases with film thickness & decreases with deposition T & angle, decreases by ≈ 80 % when annealed 30 K above deposition T, -> polarization in ASW is governed by incompletely coordinated water molecules, dangling with unbalanced dipoles at internal surface of pores
Note
Hence the importanceof characterising the HB network in ASW
Structural relaxation#
[]: LDA (prepared from HDA or vapour deposited) annealing, Raman & FTIR spectroscopy, structural relaxation -> increase of local and long-range order (starting before crystallisation and not finished at onset of crystallisation) -> contradicting findings on glass transition.
2 distinct structural state ?#
Neutron diffraction: []: Neutron diffraction on low density amorphous ice (produce from high density ASW by isobaric warming or very high density ASW by isothermal compression), -> two different forms of LDA (different compression behaviour & structures (atomistic modelling -> competition between short & intermediate order & disorder)).
Structure#
To classify
- []
- []
- []: Adsorption isotherm volumetry & FTIR spectroscopy, ASW characterisation (porosity, SSA, crystallisation), annealing induced modifications, number of surface sites decreases before crystallisation, non-microporous ice can have large specific surface area.
Warning
Good article, to read.
Fig. 11 []#
Structure factor#
Note
To define (ROO)
[] oxygen-oxygen structure factor SOO(Q) peak of liquid water shows unusual doublet structure (shifting with pressure), limits correspond to peak positions of low/high density ASW -> polyamorphism of H2O, position determined by nearest-neighbour separation of voids in spatial distribution of oxygen atoms
Variation w/ experimental conditions#
Deposition angle#
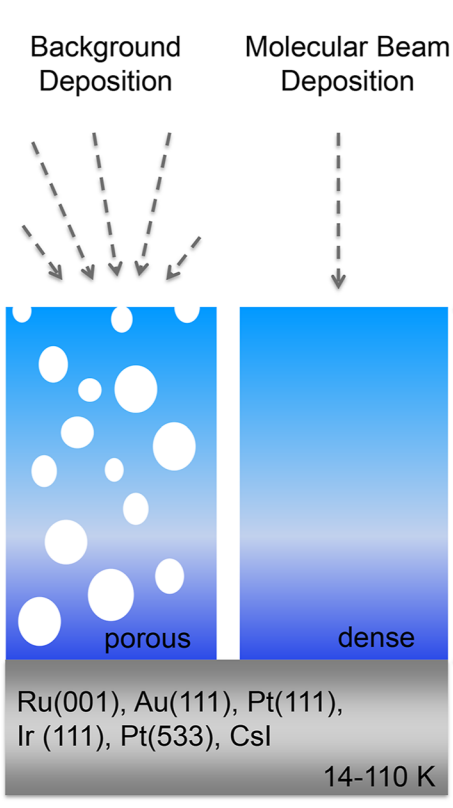
- []: ASW characterisation (laser optical interferometry): film thickness & density of ASW films (vapour deposited @ 22 K, collimated molecular beam, angle varied between normal & oblique incidence), normal incidence films presumed to be compact (0.94 g/cm3), glancing incidence ρ = 0.16 g/cm3 (> 80 % porosity), in agreement with ballistic deposition simulations.
[]
Surface#
[]: ASW & crystalline H2O ice (vapour deposited), < 100 nm – 5 μm thickness, FT-RAIRS, Al & Au substrates (similar results for both), optical effects (surface suppression/vibrational mode enhancement) vary with thickness, spectral simulations (Fresnel model)
Phase transition between polyamorphs#
Occur in a very narrow temperature-pressure interval (more info) and is characterized by sudden, step-like changes in properties such as:
- Density
- Coordination number
- Isothermal compressibility
Furthermore, tha transitions can be reversed with hysteresis, (typical from first order transition)
Note
define the different type of transitions.