is Amorphous
Contents
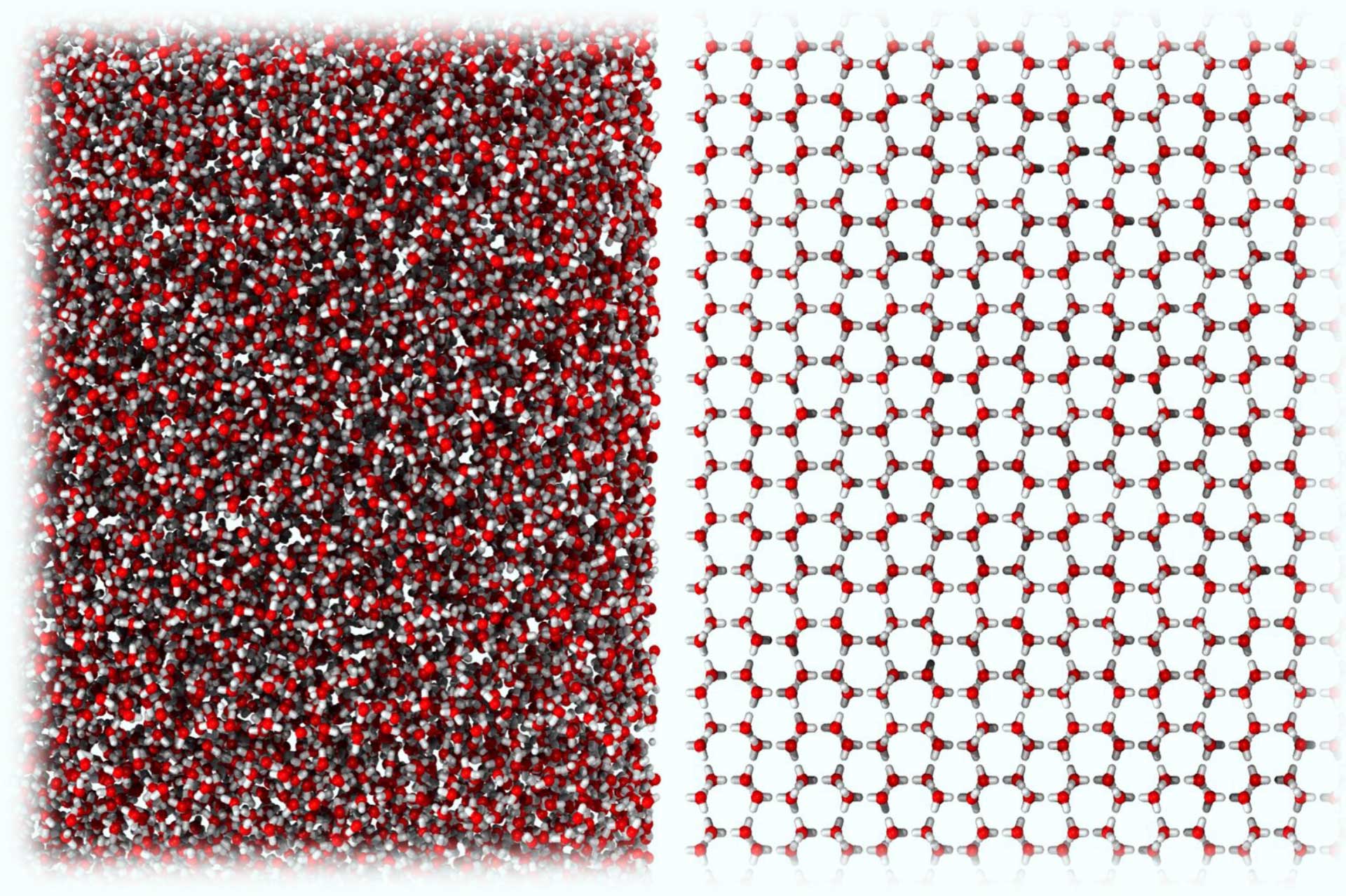
is Amorphous#
Plan
Amorphous Ices#
Litterature#
Study Title Year “Infrared spectra of amorphous solid water” by G.A. Baratta and J.A. Westley 1989 “Infrared spectra of amorphous solid water: Implications for interstellar and cometary ice” by L.D. Deshmukh et al. 2004 “H2O ice in the interstellar medium: a comparison between observed and simulated infrared spectra” by C.A. Boogert et al. 2015 “Ice grain morphology and infrared spectra in protoplanetary disks” by M. Min et al. 2016 “The Low-Temperature Infrared Spectrum of Amorphous Solid Water: A Spectroscopic and Theoretical Study” by J. P. Lewis et al. 2000 “Mid-infrared spectroscopy of interstellar ice analogs” by J.M. Brown et al. 2007 “A laboratory study of the mid-infrared spectra of amorphous solid water and water-rich ices” by A.G.G.M. Tielens et al. 2008 “Spectroscopic properties of amorphous solid water: a review” by P. Ehrenfreund et al. 2015
Review#
Review [Angell, 2004]
Bulk Organisation#
Definition#
Experiments#
Variation of the deposition angle
Controlling the Morphology of Amorphous Solid Water
Deposition temperature variations
Annealing processes
Porosity#
Definition#
Controlling the
Experiments#
Different types
Insert here table of different groups:
NASA Ames: Mastrapa, Sandford
Is data available, yes
Infrared Spectroscopy#
Key Papers:
Mastrapa (2008, 2009)
Jenny
What did we leran#
ASW is porous
3 µm band is changing when the T is increased
Water ice IR Band assignement taken from [Mastrapa et al., 2009]
Crystaline (cm -1) |
Amorphous (cm -1) |
Assignment |
A values (cm/molecule) |
|---|---|---|---|
840 |
802 |
𝜈R [Ockman, 1958] |
2.8 × 10-17 |
1650 |
1660 |
𝜈2 [Ockman, 1958] |
1.0 × 10-17 |
2266 |
2220 |
𝜈2 + 𝜈R [Whalley, 1977] |
3.3 × 10-18 |
3150 |
3191 |
𝜈1 in phase [Whalley, 1977] |
|
3220 |
3253 |
𝜈3 TO [Whalley, 1977] |
1.7 × 10-16 |
3380 |
3367 |
𝜈3 LO, 𝜈1 out of phase [Whalley, 1977] |
to cite:
d [Ockman, 1958]
e [Whalley, 1977]
Note
TO, LO, what does this mean ?
Insert own crystaline and amorphous spectra with different asssignments vertical bar
Link with Optical Constants
Real and imaginary part (how do they relate to what we observe in space)
Differential Scanning Calorimetry#
tg at 136K (ramp 30K.min-1) –> Increase in heat capacity (lower than other amorphous solid: similar to SiO2 - GeO2) –> Imply limited number of entropically different configurational states in the fluid state following tg
Dielectric relaxation behaviour expected to be determined by two processes both involving a thermaly activated dipolar reorientation:
translational-rotational diffusion of water molecules if the whole H-Bonded network relaxed within limitation of entropically different configurations
rotational diffusion of water molecules within a fixed H-Bonded structure as breaches of the Bernal-Fowler rules. –> 2 kinds of Bjerrum defects
Scattering
ASW at 140K - true glass or very viscous liquid ? What is the difference
Crystalisation#
At molecular scales (few molecules up to 100 ?) - Matrix isolation techniques

Desorption#
Porosity#
Is it Amorphous ?#
Fig. 11 Source#