Microscopy#
Optical Microscopy
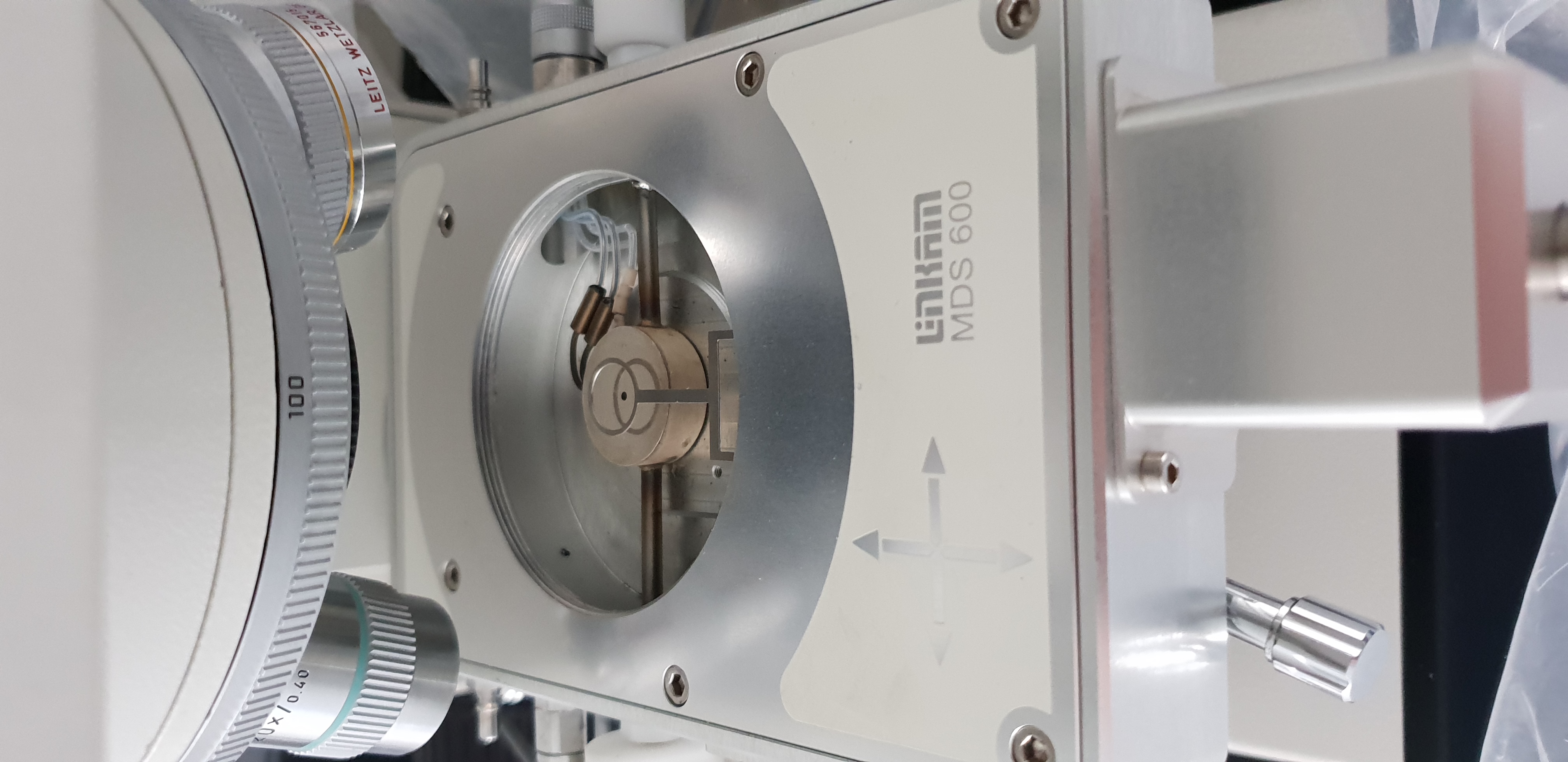
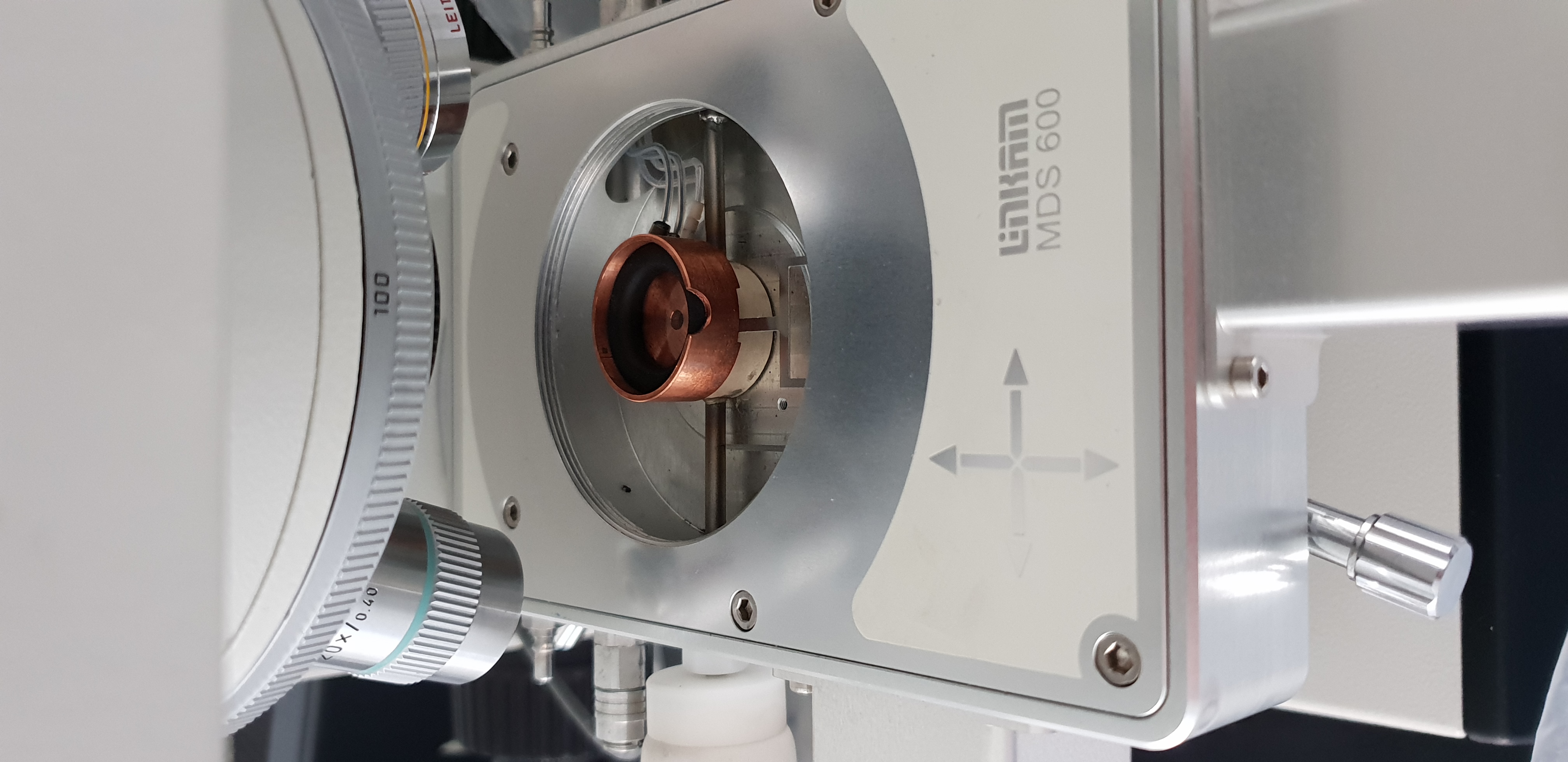
What I would like to stress out here is that I have tried to do the science I needed with what we had available (Optical microscoy with cold plate). This technique is not well suited for amorphous ice (and the experiments done have used crystaline ices, produced in liquid Nitrogen) - ie we had to make compromises. I somehow found a solution that allowed us to pour the liquid Nitrogen + ice mixture into the container you see in Figure 7, cooled by the plate you see in figure 6, but it was very difficult for the following reasons:
We have to pour the ice/liquid nitrogen mixture within the “cell”, the mixture is very turbulent due to LN2 evaporation and make the observation very difficult (unreliable focus …)
The experiment are performed at ambiant pressure so lots of condensation (impurities)
Even though the Ice container could be cool to liquid Nitrogen temperature, it is not sure that the ice is at this temperature (and liquid droplets can be seen on figure 5)
A cryostage would greatly improve my observations
Scanning Electron Microscopy ?
Abbreviated SEM
Cryo-SEM#
Cryo Electron microscopy would be of incredible help by offering:
A dedicated, state of the art, experimental setup to maintain the particles at cold temperature after they have been produced, and during their characterisation.
This is mandatory if I want to properly caracterise the particles in their amorphous state.
The particles are produced in ethane and are stored in cryo-vials.
For safety reasons, having a controled atmosphere within the SEM, would help in safe sample preparation
Shape and size distribution as well as unveiling the processes at play during the aggregation phase, like [Gundlach et al., 2018] could be achieved with a cryo SEM