Water#
Even though water is a relatively simple molecule, the third most abundant molecule in the Universe (after H2 and CO), and the most abundant molecule on Earth, it’s science is extremely complicated, let’s have a look
Topics
Going up in scale#
» Water molecule
Atomic formula
Water is a tri atomique molecule made of 1 Oxygen et 2 Hydrogens.
Molecular structure
OH covalent bonds
Fig. 20 Explain: source Wikimedia#
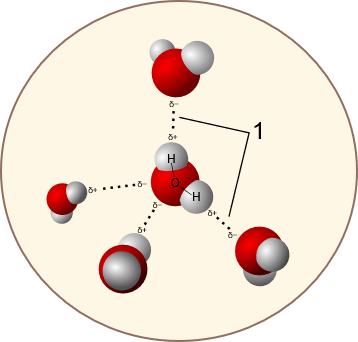
Geometry: HOH angle = 104.48 deg (Hoy 1979) - different than in tetrahedral configuration (109.47). Each molecule is electrically neutral but also polar due to the Oxygen atom having a higher electronegativity than the two Hydrogens. Length
Electron density: The Oxygen has 2 lone pair of electrons.
Strength
Oxygen lone electron pairs
Fig. 21 Explain: source Wikimedia#
tetraedral
Note
- The lone electron pair may not be similar (2s vs 2p orbitals), unless they are degenerated.
How would that affect the strength (geometry) of the Hydrogen bonds ?
Molecular Orbitals for water
Properties
Water is known for it’s anomalous properties including a So let’s have a look to it’s molecular properties to see how they arise
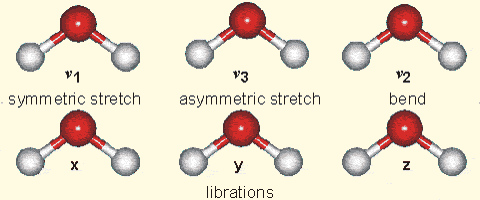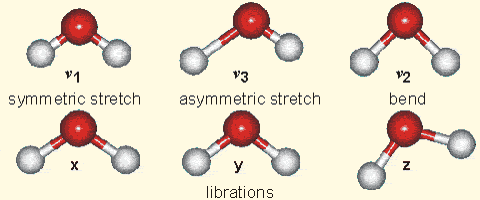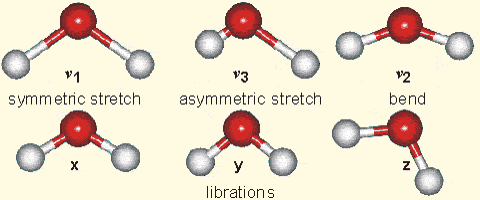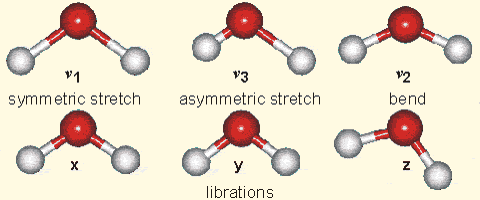
Molecular vibration
Hydrogen bonding
One of the most crucial propertie of water is it’s ability to form Hydrogen bonds (with itself or other molecules/atoms)
Note
- How is Hydrogen Bonding affecting the molecular vibration shown above is one of the key research questions we are trying to adress
Solvatation
More ?
Phase Diagram
Water can exist in all three 3 physical states under Earth conditions. We are quite familiar with liquid water, however I would like to point your attention on the solid phase of water, ice. Many polymorphs, the one you know being Hexagonal ice (Ih).
Phase lines on this phase diagram represents a phase boundary and gives the conditions when two phases may stably coexist in any relative proportions (having the same Gibbs free energy and identical chemical potential)
Fig. 25 Explain: source Wikimedia#
Water models
Now we have seen some of water properties let’ have a look at how scientists model the water molecule to push further their investigations. A key point is that:
- There is no universal water model that can explain all the water properties
[] reviewed the different models created over time (up to 2015) and classified them in 4 groups:
- 1
- 2
- 3
- 4
Fig. 26 Explain: source []#
The Lennard-Jones relationship
- Models for individual molecules to perform Molecular Dynamics simulations.
» Gas
Gas: weakly interacting molecules
Thermodynamic properties
» Cluster
Hydrogen Bonding
With two other water molecules
- DDAA
- …
Small clusters
Large clusters
Network Description
How do we describe an assembly of water molecules
- Protonated Water Cluster (PW)
Modeling Clusters
Thole-type model
- 1999
- 2002
- Hydrogen-bond pattern to characterize water network
- The dipole moment of a constituent molecule in a water cluster is enhanced depending on the local HB network around the water molecule
» Liquid
Note
- description of the science in liquid water …
- Low T vs High T liquid - subject of polemic
Constant breaking and reorganisation of individual hydrogen bonds on a picosecond timescale. However
Ice#
» Overview
Ice rules (or Bernal–Fowler rules): basic principles that govern arrangement of atoms in water ice
- Each oxygen is covalently bonded to two hydrogen atoms
- The oxygen atom in each water molecule forms two hydrogen bonds with other water molecules
Hexagonal Ice
Ih
» Water ice polymorphism
» Amorphous vs Crystaline Ice
Comparison
Crystaline
- Polymorph: 18 ?
- Ice rules
Amorphous
- Polymorph: 5
Amorphous Solid Water
» Crystalisation
At molecular scales (few molecules up to 100 ?) - Matrix isolation techniques